Volume 11, Issue 4 (12-2021)
J Health Saf Work 2021, 11(4): 556-576 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khoshakhlagh A H, Golbabaei F, Beygzadeh M, Shahtaheri S J. Study of the dynamic adsorption process of toluene by a microporous copper metal-organic framework. J Health Saf Work 2021; 11 (4) :556-576
URL: http://jhsw.tums.ac.ir/article-1-6574-en.html
URL: http://jhsw.tums.ac.ir/article-1-6574-en.html
Amir Hossein Khoshakhlagh1 
 , Farideh Golbabaei2
, Farideh Golbabaei2 
 , Mojtaba Beygzadeh3
, Mojtaba Beygzadeh3 
 , Seyed Jamaleddin Shahtaheri *
, Seyed Jamaleddin Shahtaheri * 
 4
4

 , Farideh Golbabaei2
, Farideh Golbabaei2 
 , Mojtaba Beygzadeh3
, Mojtaba Beygzadeh3 
 , Seyed Jamaleddin Shahtaheri *
, Seyed Jamaleddin Shahtaheri * 
 4
4
1- Department of Occupational Health Engineering, Faculty of Health, Kashan University of Medical Sciences, Kashan, Iran
2- Department of Occupational Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
3- Department of Energy, Materials & Energy Research Center, Tehran, Iran
4- Department of Occupational Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ,shahtaheri@tums.ac.ir
2- Department of Occupational Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
3- Department of Energy, Materials & Energy Research Center, Tehran, Iran
4- Department of Occupational Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ,
Abstract: (1691 Views)
Introduction: Toluene is considered as a group of chemical contaminants, causing problems for people’s health. Due to the high rate of evaporation and rapid emission in the surrounding environment, it leads to the exposure of many employees and people at risk and, subsequently, its irreparable effects on their health in different jobs. Therefore, its removal is very important. In the present study, this contaminant was removed using the copper metal-organic framework (MOF) under different operating conditions.
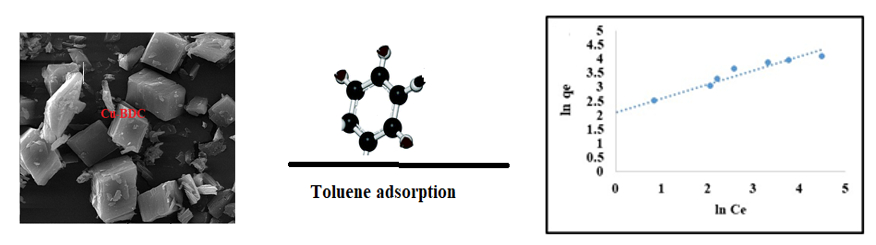
Material and Methods: In this study, the copper MOF was synthesized using the one-pot and in situ method. Physical and morphological properties of the adsorbent were investigated using BET, XRD, FTIR and SEM techniques. The efficiency of the adsorbent in removing toluene from the air stream under the dynamic adsorption system was investigated by examining the effect of the variables of adsorbent mass, pollutant concentration and humidity. Isotherm, thermodynamics and kinetics equations were used to evaluate the data.
Results: The results of experiments determining the properties of the metal-organic framework showed the formation of pure Cu-BDC crystals with mean and particle size distribution of 1.95 nm. The specific surface area calculated by the BET method for the mentioned sample was 686 m2 g-1 and the total volume of structural pores was 0.335 g3 cm3. The presence of micropores increased the dynamic adsorption capacity of toluene. The findings follow the Langmuir isotherm model and the Pseudo-second order kinetic model. Based on the results of thermodynamic studies, entropy change (ΔS°) and enthalpy change (ΔH°) were equal to -0.44 kJ mol-1 K-1 and -15.67 kJ mol-1, respectively. Gibbs free energy change (ΔG°) was also calculated negatively, indicating that the adsorption process was spontaneous and exothermic. The regeneration of the adsorbent was 77% after three cycles.
Conclusion: According to the results of this study, the microporous copper MOF can be used as a result of cheapness, high access, high adsorption capacity and appropriate regeneration rate in different operating conditions for adsorption of toluene.
Material and Methods: In this study, the copper MOF was synthesized using the one-pot and in situ method. Physical and morphological properties of the adsorbent were investigated using BET, XRD, FTIR and SEM techniques. The efficiency of the adsorbent in removing toluene from the air stream under the dynamic adsorption system was investigated by examining the effect of the variables of adsorbent mass, pollutant concentration and humidity. Isotherm, thermodynamics and kinetics equations were used to evaluate the data.
Results: The results of experiments determining the properties of the metal-organic framework showed the formation of pure Cu-BDC crystals with mean and particle size distribution of 1.95 nm. The specific surface area calculated by the BET method for the mentioned sample was 686 m2 g-1 and the total volume of structural pores was 0.335 g3 cm3. The presence of micropores increased the dynamic adsorption capacity of toluene. The findings follow the Langmuir isotherm model and the Pseudo-second order kinetic model. Based on the results of thermodynamic studies, entropy change (ΔS°) and enthalpy change (ΔH°) were equal to -0.44 kJ mol-1 K-1 and -15.67 kJ mol-1, respectively. Gibbs free energy change (ΔG°) was also calculated negatively, indicating that the adsorption process was spontaneous and exothermic. The regeneration of the adsorbent was 77% after three cycles.
Conclusion: According to the results of this study, the microporous copper MOF can be used as a result of cheapness, high access, high adsorption capacity and appropriate regeneration rate in different operating conditions for adsorption of toluene.
Type of Study: Research |
Received: 2021/12/24 | Accepted: 2021/12/31 | Published: 2021/12/31
Received: 2021/12/24 | Accepted: 2021/12/31 | Published: 2021/12/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |